08.03Non-Science in Dating the Earth
May 2004
Chuck Roche, PhD
One topic for skeptics involves the age of the earth. Many creationists have argued for a young earth, one less than 15,000 years, while others allow for just a few million years. Most do not realize the scientific community is rather unified on the earth being several billion years in age. The most straightforward dating technique is emphasized in this paper – radiometric dating.
Radiometric dating
The process of determining the age of rocks from the decay of their radioactive elements has been in widespread use for over half a century [1]. There are over forty such techniques, each using a different radioactive element or a different way of measuring them. It has become increasingly clear that these radiometric dating techniques agree with each other and as a whole, present a coherent picture in which the earth was created billions of years ago. Many are completely unaware of the great number of independent, laboratory measurements that have shown these methods to be consistent.
Rocks are made up of many individual crystals, and each crystal is usually made up of at least several different chemical elements such as iron, magnesium, and silicon. Most elements in nature are stable and do not change into other elements. Some atoms eventually change from one element to another by a process called radioactive decay. If there are many atoms of the original element, called the parent element, the atoms decay to another element, called the daughter element, at a predictable rate. The passage of time can be charted by the reduction in the number of parent atoms, and the increase in the number of daughter atoms. When all the atoms of the radioactive element are gone, the rock no longer keeps time.
If it takes a certain length of time for half of the atoms to decay, it will take the same amount of time for half of the remaining atoms, or one-fourth of the original total, to decay. In the next time interval, with only one-fourth remaining, only one eighth of the original total will decay. By the time ten of these intervals, or half-lives, have passed, less than one thousandth of the original number of radioactive atoms is left. There is no way to change the rate at which radioactive atoms decay.
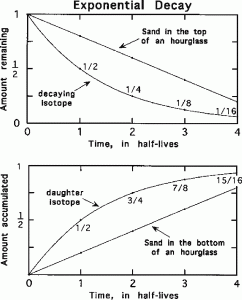 In, Figure 1, the rate of loss of sand from the top of an hourglass compared to the exponential type of decay of radioactive elements. Most processes we are familiar with are linear, like sand in the hourglass. In exponential decay, the amount of material decreases by half during each half-life. As shown in the bottom panel, the daughter element or isotope amount increases rapidly at first, then more slowly with each succeeding half-life.
In, Figure 1, the rate of loss of sand from the top of an hourglass compared to the exponential type of decay of radioactive elements. Most processes we are familiar with are linear, like sand in the hourglass. In exponential decay, the amount of material decreases by half during each half-life. As shown in the bottom panel, the daughter element or isotope amount increases rapidly at first, then more slowly with each succeeding half-life.
A partial list of the parent and daughter isotopes and the decay half-lives are given in Table 1. Notice the large range in the half-lives. Isotopes with relatively short half-lives are useful for dating correspondingly shorter intervals, and can usually do so with greater accuracy, just as you would use a stopwatch rather than a grandfather clock to time a 100-meter dash.
Table 1. Radiometric Isotopes and their Half-lives
| Radioactive Isotope (Parent) | Product (Daughter) |
Half-Life (Years) |
| Samarium – 147 | Neodymium – 143 | 106 billion |
| Rubidium-87 | Strontium-87 | 48.8 billion |
| Rhenium-187 | Osmium-187 | 42 billion |
| Lutetium-176 | Hafnium-176 | 38 billion |
| Thorium-232 | Lead-208 | 14 billion |
| Uranium-238 | Lead-206 | 4.5 billion |
| Potassium-40 | Argon-40 | 1.26 billion |
| Uranium-235 | Lead-207 | 0.7 billion |
| Beryllium-10 | Boron-10 | 1.52 million |
| Carbon-14 | Nitrogen-14 | 5715 |
The half-lives have all been measured directly, either by using a radiation detector to count the number of atoms decaying in a given amount of time from a known amount of the parent material, or by measuring the ratio of daughter to parent atoms in a sample that originally consisted completely of parent atoms. Radiometric dating began 100 years ago, but progress was relatively slow before 1940. For many of the dating techniques, we now have had fifty years over which to measure and remeasure half-lives. Very precise counting of the decay events or the daughter atoms can be done, so that while the number of, for example, rhenium-187 atoms decaying in 50 years is a very small fraction of the total, the resulting osmium-187 atoms can be very precisely counted.
Rocks formed from molten lava are referred to as Igneous rocks and are good candidates for dating. When the molten material cools and hardens, the atoms are no longer free to move about. Any daughter atoms from radioactive decays occurring after a rock cools are trapped where they are made within the rock. These atoms are like the sand grains accumulating in the bottom of the hourglass. To determine the age of the rock, the number of daughter atoms and the number of remaining parent atoms are measured, and use the half-life to calculate the time it took to make those daughter atoms.
Potassium-Argon. Potassium is an abundant element in the Earth’s crust. One isotope, potassium 40, is radioactive and decays to two different daughter products, calcium-40 and argon-40, by two different decay methods. This is not a problem because the production ratio of these two daughter products is precisely known, and is always constant: 11.2% becomes argon-40 and 88.8% becomes calcium-40. It is possible to date some rocks by the potassium-calcium method, but this is not often done because it is hard to determine how much calcium was initially present. Argon, on the other hand, is a gas. Whenever rock is melted to become magma or lava, the argon tends to escape. Once the molten material hardens, it again begins to trap the argon produced from its potassium. In this way the potassium-argon clock is clearly reset when an igneous rock is formed.
In its simplest form, the geologist simply needs to measure the relative amounts of potassium-40 and argon-40 to date the rock. The age is given by a relatively simple equation:![]()
where t1/2 is the half-life, and ln is the natural logarithm.
However, some may argue a small amount of argon remains in a rock when it hardens. This is usually trapped as very tiny air bubbles in the rock. Scientists allow for it. This would most likely be the case in either young rocks that have not had time to produce much radiogenic argon, or in rocks that are not abundant in potassium. One must have a way to determine how much air-argon is in the rock. This can be done because air-argon has a couple of other isotopes, the most abundant of which is argon-36. The ratio of argon-40 to argon-36 in air is well known, at 295. Thus, if one measures argon-36 and argon-40, one can calculate and subtract off the air argon-40 to get an accurate age.
One of the best ways to show an age-date is correct is to confirm it with other dating techniques. Although potassium-argon is one of the simplest dating methods, there are still other cases where it does not agree with other methods because the gas within bubbles in the rock is from deep underground rather than from the air. This gas can have a higher concentration of argon-40 and is called parentless argon-40 because its parent potassium is not in the rock being dated, and is also not from the air. In these unusual cases, the date given by the normal potassium-argon method is inaccurate. Scientists solved this problem forty years ago with the argon-argon method.
Argon-Argon. This method uses exactly the same parent and daughter isotopes as the potassium-argon method. In effect, it is a different way of telling time from the same clock. Instead of simply comparing the total potassium with the non-air argon in the rock, this method can tell exactly what and how much argon is directly related to the potassium in the rock.
In the argon-argon method, the rock is placed near the center of a nuclear reactor for a number of hours. A nuclear reactor emits a very large number of neutrons, which can change a small amount of the potassium-39 into argon-39. Argon-39 is not found in nature because it has a 269-year half-life (the shortness of this half-life doesn’t affect the argon-argon dating method as long as the measurements are made within about five years of the neutron dose). The rock then is heated in a furnace to release both the argon-40 and the argon-39 (representing the potassium) for analysis. The heating is done at incrementally higher temperatures and at each step the ratio of argon-40 to argon-39 is measured. If the argon-40 is from decay of potassium within the rock, it will come out at the same temperatures as the potassium-derived argon-39 and in a constant proportion. If there is some excess argon-40 in the rock, it will cause a different ratio of argon 40 to argon-39. A good argon-argon age determination will have many heating steps which all agree with each other.
Earth rocks, moon rocks, and meteorites
Earth rocks have been dated at up to about 3.9 billion years. But actually only a very small portion of the earth’s rocks are that old. From satellite data we know that the earth’s surface is constantly rearranging itself little by little as earthquakes and volcanic activity occur. Remelting is common and over billions of years, none of the rocks may have survived from the creation of the earth without undergoing remelting, metamorphism, or erosion; scientist can conclude from this line of evidence is that the earth is at least 3.9 billion years old.
When scientists began systematically dating meteorites, they learned nearly all of the meteorites had practically identical ages – 4.56 billion years. These meteorites are chips off asteroids that went from very hot to cool in a relatively short period of time, excellent candidates for independent dating studies. The meteorites have not been remelted ever since, so the ages have generally not been disturbed. Contrary to a few wackos, we have visited the moon, and gathered rocks. The oldest rocks we have from the moon do not exceed 4.1 billion years, though a larger sampling might yield some slightly older ages.
Most scientists agree that all the bodies in the solar system were created about the same time. There is evidence from the uranium, thorium, and lead isotopes that links the earth’s age with that of the meteorites. This is why the scientific community puts the earth to be about 4.5-4.6 billion years old.
Comments
There are more than forty different radiometric dating methods being used by several hundred laboratories acting independently around the world and they are in agreement. The differences found in the scientific literature are nearly always less than 2%, not the orders of magnitude needed to allow for a young earth.
Decay rates have been directly measured over the last 50-80 years. In some cases a batch of the pure parent material is weighed and set aside for a long time, and then the resulting daughter material is weighed. We have compelling evidence; tests repeated 80 years later result in dating the rock to be 80 years older. This represents an ideal scientific methodology in that it includes proven physical laws, hypothesis, measurement, independent repeatability, and as 80 years pass by, reconfirmation of a datum point 80 years greater than the first measurement.
Bible-Based technique
Although the Bible never mentions the earth’s age, some people have tried to calculate the date of creation by adding up the life spans of the generations listed in Genesis chapters 5 and 11. A literal interpretation of the week of creation, even if some generations were left out of the genealogies, the earth would be less than ten thousand years old.
The Bible records that Jesus converted water into wine, and one may infer the wine appeared aged even though it was new. And when God created trees, perhaps they had rings implying years of age even though they were new. One can ponder if Adam and Eve were created with belly buttons or cavities.
Extending this concept to creation of the modern earth in six days, God may have created evidence that the earth and universe were billions of years old. However, considering this for the entire universe makes God appear to be the most deceitful of beings. To put sediment layers on sediment layers of gradual fossil evolution confirmed by the rocks found near the fossils would be physical evidence to doubt creationism or a young earth. Furthermore, this concept would require consistent deceit in every aspect of nature, whether it be half-lives of elements or light from distant stars. If God were to perpetrate such a fraud, one could not trust any ancient history regarding, for example, the great pyramids or the Dead Sea Scrolls, and astronomy would be a pseudoscience.
The Age of the Moon from the Depth of Moon Dust [2, 3]
This argument relies on hypotheses made by scientists R. A. Lyttleton in 1956 and Hans Petterson in 1960. According to their estimates, if the moon were billions of years old, dust at least 100 feet deep would have accumulated on the surface of the moon, too deep for moon landings. Lyttleton and Petterson’s estimates were never published in a major journal, and were never peer reviewed.
If NASA engineers believed the dusty theory and the moon to be billions of years old, why would they not design space suits air conditioning prepared for lots of dust, ‘snow shoes’ to walk on dust, landing instrumentation to land on soft dust, and so forth? Because the overwhelming bulk of the scientific community believed the moon had very little dust. Choosing to quote the exception to mainstream science is an aspect of pseudoscience or ignorance. Extending those postulates to real world events often points to a glaring contradiction – if they were true, why did so many of the best engineers ignore the evidence?
The Ocean Salt Theory
Scientists have not used the salt level in the oceans as a measure of the age of the earth but some creationists have. The argument is summarized in a creationism web site [4] as:
“About 250 million metric tons [25 x1010 kg/yr] of salt is dumped into the oceans by rivers every year, and about 75 million tons is removed every year by absorption, evaporation and a few other processes. That leaves 175 million tons of salt added to the oceans every year. The salt levels in the ocean today confirm an earth less than 100 million years old; any older and there would be too much salt.”
Sharp skeptics can quickly see the fallacy of this argument. If for the last few hundreds years, the amount of salt in the ocean has been dramatically increasing, then it would be a much bigger news story than global warming – an argument about a temperature increase of less than 1°F for the last hundred years. All sea life would be dead very soon. Scientists are monitoring salt density as a function of time but do not see it as a tool to estimate the age of the earth. When one reviews geological scientific texts, a better picture is observed.
Removal of salt from the oceans comes from the following naturally varying processes in descending order: high-T brine alteration, sea spray, halite deposition, cation exchange, burial of pore water, low-T brine alteration, biogenic carbonate formation, zeolite formation, and biogenic silica formation [5]. Each remover has estimated tolerance and the sum total is approximately 30×1010 kg/yr. Creationists lacking a geological science background and attention to numerical detail claim all kinds of numbers or have been misled by prior creationist authors.
Scientists put the input and output nearer a balance, about 30×1010 kg/yr and do not maintain it is exact. They monitor the mineral changes in salt water and should not use it as a tool to date the earth.
Comments
Many scientific concepts cannot be used to date the earth. Therefore, scientists may ignore such concepts for dating but they become fodder for some creationists or conspiracy theorists. Extending the logic of some young earth evidence just a few hundreds years would make clear the earth has little time left. Clarifying the science purported by creationists often contradicts what the thousands of NASA engineers believe and do.
The good news is time will tell. We’ll continue to monitor nature. We’ll test our physical laws and assumptions. When NASA does panic about an unknown, it represents an opportunity to learn. Scientific beliefs are literally under a microscope and will be discarded if demonstrably false. Perhaps at the other extreme are religious beliefs, ones which attempt to remain rigid in the face of new evidence.
References
1) R.C. Weins, Radiometric Dating, A Christian Perspective; http://www.asa3.org/ASA/resources/Wiens.html.
2) Hans Petterson, “Cosmic Spherules and Meteoric Dust,” Scientific American, 202:132.
3) http://www.cincinnatiskeptics.org/blurbs/
4) http://www.parentcompany.com/creation_essays/essay18.htm
5) http://www.glenn.morton.btinternet.co.uk/salt.htm
6) A bulk of the paper is taken from: R.C. Weins, Radiometric Dating, A Christian Perspective; http://www.asa3.org/ASA/resources/Wiens.html.
7) http://www.cincinnatiskeptics.org/blurbs/
8) http://www.parentcompany.com/creation_essays/essaytoc.htm
